Research Interests
Dr. Ho’ research has revolved around three major areas – identification of specific compounds in raw and processed food, advancing the understanding of Maillard chemistry and human diseases, and identification and molecular mechanism studies of bioactive natural products.
Project 1: Determination of Key Aroma Active Compounds In Raw And Roasted Lily Bulbs (Bai He) – An Ingredient In Chinese Cuisine
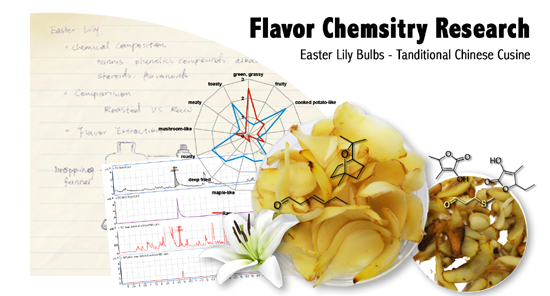
Lily bulbs, known as ‘Bai He’ in Chinese, are a popular ingredient used in Asian cooking, primarily in stir-fried dishes, soups, and stews. Despite their popularity, little is known about the odorants responsible for their pleasant aroma. The key aroma active compounds present in both raw and roasted lily bulbs (Lilium longiflorum Thunb.) were isolated by solvent extraction followed by solvent-assisted flavor evaporation (SAFE) and gas chromatography/Olfactometry (GC/O) analysis. The results of a comparative aroma extract dilution analysis (cAEDA) in the flavor dilution (FD) factor range from ≥ 1 to 1024 resulted in 48 aroma-active compounds, all of which were reported in L. longiflorum bulbs for the first time. The highest FD factors in raw lily bulbs were determined for (E)-hex-3-enal (green, FD 1024) and 3-(methylsulfanyl)propanal (methional) (cooked potato-like, FD 1024) followed by 1,8-cineole (eucalyptol) (eucalyptus-like, FD 256) and 2-phenylacetaldehyde (floral, FD 256). After thermal treatment, the odorants with high FDs in raw lily bulbs decreased in intensity. As a result of the roasting process, an increase in and generation of 34 aroma-active compounds was found in the roasted lily bulbs. The aroma-active compounds with FD factors ≥ 1024 in roasted lily bulbs were identified as 2-acetyl-1-pyrroline (roasty), 3-(methylsulfanyl)propanal (methional) (cooked potato-like), 2-ethyl-3,5-dimethylpyrazine (earthy), 2,3-diethyl-5-methylpyrazine (earthy), 3-hydroxy-4,5-dimethylfuran-2(5H)-one (sotolon) (maple-like), 5-ethyl-3-hydroxy-4-methyl-5H-furan-2-one (abhexon) (maple-like) and 2-methoxy-4-[(E)-prop-1-enyl]phenol ((E)-isoeugenol) (clove-like). In summary, most of the aroma-active compounds with high FD factors in raw lily bulbs decreased significantly as a result of thermal treatment and a new pool of aroma-active compounds were formed during the roasting process.
Project 2: Potential Role of Methylglyoxal on Parkinson's Disease --- the Reaction between Methylglyoxal and Dopamine
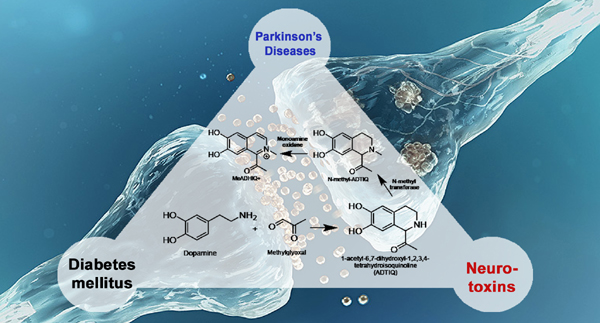
About one and a half million people are affected by Parkinson’s disease (PD) every year. It is a neurodegenerative disorder on dopaminergic neurons in the substantia nigra, with primary effects on motion disorder. The causes for the Parkinsonism are generally divide into genetic factors like mutation and environmental factors like heavy metals as well as some endogenous or exogenous agents. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and other MPTP-like neurotoxins such as salsolinol are well known and considered to cause Parkinsonism due to their in vivo metabolic products’ specific toxicity for dopaminergic neurons.
Recent studies showed that the diabetes patient is more likely to develop the Parkinson’s disease. It has been suspected that the methylglyoxal, one metabolic product of glycolysis, is associated with this phenomenon because the body concentration of methylglyoxal for diabetic patients will be three to six times higher than healthy people. The dopamine-derived tetrahydroisoquinoline (TIQ), 1-acetyl-6,7-dihydroxyl-1,2,3,4-tetrahydro- isoquinoline (ADTIQ), has been detected in frozen brain tissue of human with Parkinson’s disease. It can be produced by the reaction of dopamine and methylglyoxal in physiological condition and has been regarded a novel endogenous neurotoxin.
In our study, in addition to ADTIQ, 6,7-dihydroxy-1,2,3,4-tetrahydro- isoquinoline or called norsalsolinol was also detected in the reaction system of dopamine and methylglyoxal by LC-MS/MS. And based on the Pictect-Spengler reaction, there are two regioselectivities for the products under neutral conditions. So there are two isomers for both ADTIQ and norsalsolinol. Additionally, according to the proposed reaction mechanism, the norsalsolinol was generated from ADTIQ by deacylation. On the other hand, with different ratios of dopamine and methylglyoxal, different levels of reaction under the physiological condition were also monitored by HPLC-UV. It revealed that when the ratio of dopamine and methylglyoxal is over 1:10, the reaction could almost be completed within 24 hours.
In conclusion, we showed for the first time that the potential neurotoxin norsalsolinol could be generated through the reaction of dopamine and methylglyoxal. And also can be one reason why the norsalsolinol was detected in the frozen brain of humans.
Project 3: Identification and Quantification of Potential Anti-inflammatory Hydroxyciannamic Acid Amides from Lycium barbarum
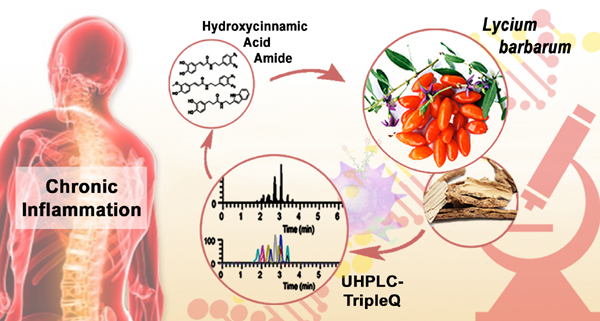
Hydroxycinnamic acid amide (HCAA) are commonly found in flowering plants, when conjugation of cinnamate derivatives with tyramine, tryptamine and dopamine derivatives. A large number of studies have reported various biological activities exhibited by the HCCA family, including anti-fungal, antioxidant and anti-cancer properties. Lycium barbarum belongs to the genus Lycium, distributing throughout temporal area of Northern-Central and Western China, South America and South Africa. The fruits, wolfberries, were commonly consumed as functional foods in order to enhance their positive effects on human health. In addition, the leaves, Folium Lycii were also included as traditional medicinal remedy. In this study, we synthesized a series of hydroxycinnamic acid amide (HCCA) compounds and developed and UHPLC-MS/MS method for identification and quantification of the synthetic compounds in the fruits and leaves extracts. The method was fully validated, with respect to specificity, linearity, intra- and inter-day precision and accuracy, limit of detection (LOD), limit of quantification (LOQ), recovery, and reproducibility. Finally, the developed method was successfully applied to quantify nine and 10 HCCA compounds in fruits and leaves, respectively. The found HCCA compounds were reported in the fruits and leaves for the first time. The potential anti-inflammatory activities of analytes were also investigated in vitro by using LPS-activated RAW264.7 murine macrophages, elucidating the detail anti-inflammatory mechanism by reduction of nuclear transcription factor NF-κB activation and downstream protein expression, as well as inhibition of PI3K/Akt signaling pathway. These results indicated that both the fruits and leaves of Lycium barbarum demonstrated promising anti-inflammatory effects and could potentially be used to prevent and treat inflammation and inflammation-related diseases.

