Research Interests
Our laboratory studies how the metabolism of essential micronutrients, such as vitamin A and its carotenoid precursor β-carotene, influences health and disease throughout the life cycle. A major focus of our research is on maternal-fetal metabolism of vitamin A and β-carotene with the ultimate goal to understand how to prevent or improve congenital defects as well as maternal pathological conditions associated with both deficiency and excess of the vitamin. We also have a keen interest in understanding the impact of vitamin A status and intake on the development and establishment of the microbiome, which is emerging as a critical determinant of health.
β-apocarotenoids as critical regulators of mammalian embryonic development
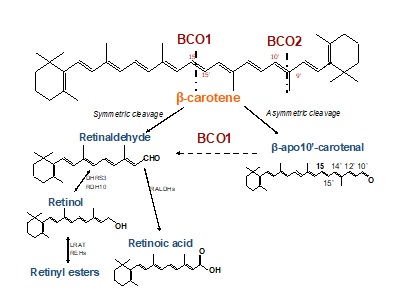
Mammalian embryonic development requires vitamin A for normal cell fate specification, patterning, and differentiation, indicating that both gestational vitamin A deficiency and excess can cause morphological defects of essentially all organs. Such activities have been attributed predominantly to the action of retinoic acid, the vitamin A metabolite that binds the nuclear retinoic acid receptors (RARs) to regulate the transcription of genes that are critical during embryogenesis. β-carotene, the most abundant dietary vitamin A precursor, is an important source of retinoids for adult and embryonic tissues. Previous studies from our laboratory have demonstrated that the symmetric β-carotene cleavage to retinaldehyde by the cytosolic enzyme BCO1 is a critical pathway for retinoid generation in embryonic tissues. At the same time, asymmetric cleavage of the provitamin A carotenoid by BCO2 also occurs, generating β-apo10’-carotenal (apo10AL) which, upon the action of BCO1, can yield retinoids. In adult tissues, the contribution of this latter pathway appears to be negligible. Instead, BCO2 is believed to prevent oxidative stress induced by toxic accumulation of carotenoids in mitochondria. We recently obtained evidence that BCO2 indeed plays a critical role in preventing β-carotene toxicity during early embryogenesis but also that β-apo10’-carotenal can restore normal development in a dose-dependent manner in a mouse model of severe embryonic vitamin A deficiency (Bco2-/-Rbp4-/-).
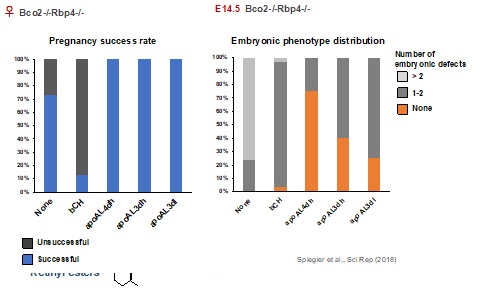
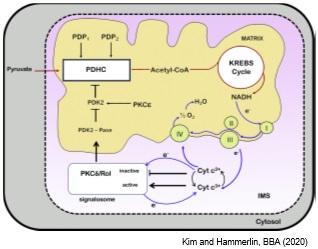
We are currently i) identifying the mechanisms of β-carotene toxicity during early embryogenesis; ii) unequivocally establishing the growth restoring function of β-apo10’-carotenal under VAD; and iii) determining whether β-apo10’-carotenal performs this activity as retinoid precursor or per se. In regard to the latter, our recent findings suggest that β-apo10’-carotenal, like retinol, influences mitochondrial function by regulating cellular respiration, thus cell survival, in a PKCδ-dependent manner. We are also investigating the role of retinol and β-apocarotenoids as regulator of the PKCδ signalosome during embryogenesis.
β-carotene and its derivatives modulate placenta lipoprotein biosynthesis
Our laboratory identified a novel feed-forward regulatory mechanism that, by modulating placental lipoprotein biosynthesis, facilitates placental-fetal transfer of β-carotene. We demonstrated that when β-carotene is available, placental Bco2 transcription increases, thus enhancing the generation of β-apo10’ carotenal from β-carotene via asymmetric cleavage. This metabolite increases the transcription of Hnf4∝, which in turn increases transcription and activity of microsomal triglyceride transfer protein (MTP) - the master regulator of intracellular assembly and secretion of apoB-lipoproteins - and decreases the transcription of CoupTFI/II, repressors of MTP. This opposing regulatory mechanisms results in enhanced transcription and activity of MTP, increased lipoprotein biosynthesis and the transfer of intact β-carotene towards the embryo. As β-apocarotenoids can function by antagonizing the activity of retinoic acid, the active form of vitamin A that acts as a transcriptional regulator, we hypothesized the existence of a retinoic acid responsive element (RARE) in the promoter of the corresponding target gene(s). Our data also suggested that a transcription factor upstream of Hnf4∝ could be the direct target of the above-described β-apo10’ carotenal activity. Further, we hypothesized that apo10AL antagonizes RA for the binding to RARs and/or RXRs on an RARE cis-element of the promoter of such factor, ultimately turning on Hnf4∝ and/or suppressing CoupTFI/II.
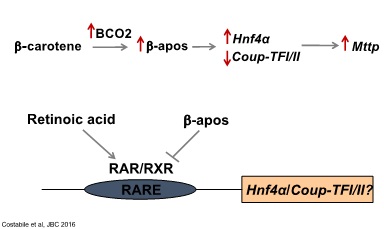
By employing in vivo and in vitro approaches we are currently investigating whether the differential generation of β-apo10’ carotenal vs. retinoic acid from β-carotene in placenta and their antagonistic transcriptional activity are indeed the key factors that optimize placental-fetal transfer of β-carotene by ultimately regulating MTP function. We are also working to identify the direct target of the β-apo10’ carotenal action as well as the full regulatory cascade that ultimate modulates placenta lipoprotein biogenesis in response to dietary intake of carotenoids. Finally, we aim at establishing whether β-apocarotenoids similarly regulate intestinal lipoprotein assembly and secretion in other organs, such as intestine.
Vitamin A and β-carotene metabolism in cardiac physiological remodeling
Vitamin A is an essential nutrient that regulates not only organs’ development but also their functions in adulthood, mainly owing to the transcriptional role of its active metabolite, retinoic acid. To date, the literature has implicated retinoic acid signaling as a cardioprotective against pathological remodeling. However, the role of retinoic acid in cardiac physiological adaptations has never been explored. We are currently investigating, both in vivo and in vitro, how cardiac alterations of vitamin A and/or β-carotene metabolism may impact such adaptations postnatally and during pregnancy, unique instances of cardiac physiological remodeling.
The microbiome of vitamin A deficiency (VAD)
Vitamin A modulates intestinal morphology and functions by maintaining epithelial barrier integrity, gut immunity and inflammation. Thus, VAD is often associated with pathological conditions of the gut. A "healthy" intestine is also critical to support a "healthy" microbiome, a complex and large microbial community residing in the gastrointestinal tract that helps maintain proper intestinal functions in a feedback loop, positively influencing the overall health status. A specific taxonomic profile of the microbiome of VAD has begun to emerge, but the relationship between dietary vitamin A content, host vitamin A status, and gut microbiome is complex and remains to be fully elucidated. By using a genetic model of VAD we aim at understanding the host-microbial interaction in the context of VAD. Our ultimate goal is the identification of unique signatures of gut microbial functional pathways that could be of diagnostic value and also drive interventions to restore normal vitamin A status and improve intestinal functions in VAD by manipulating the microbiome.